Deuterated DMSO

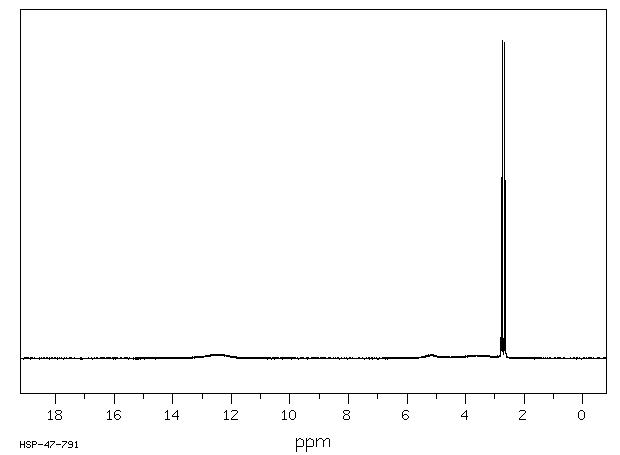
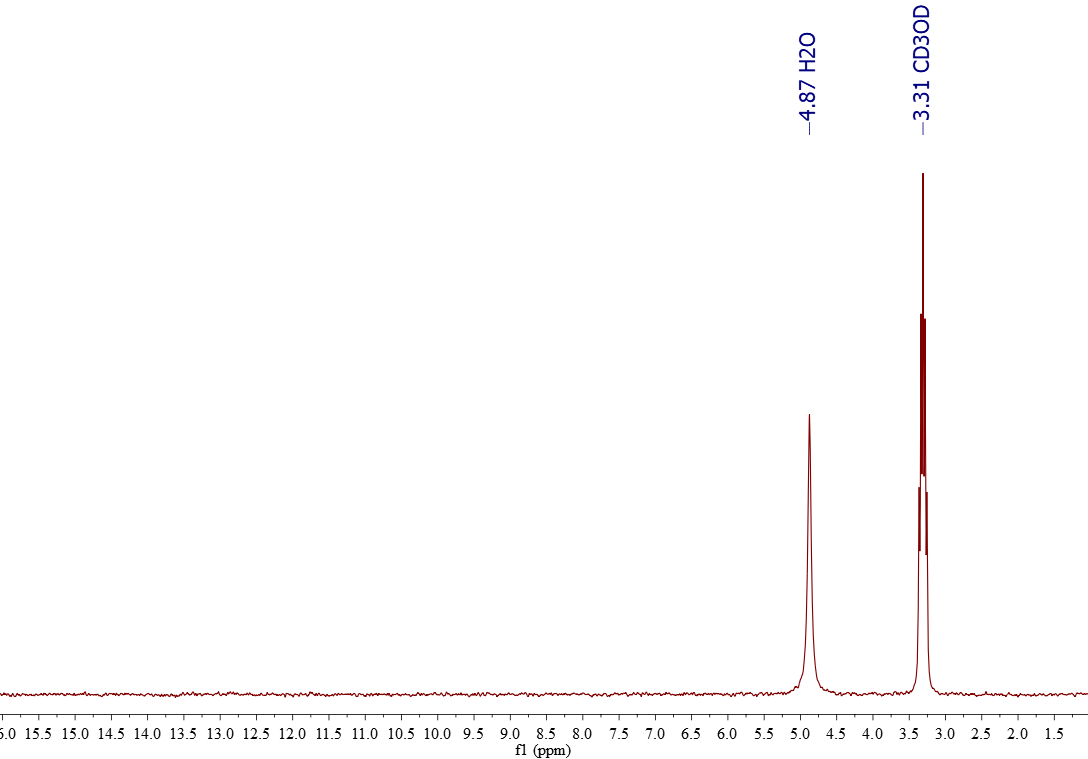
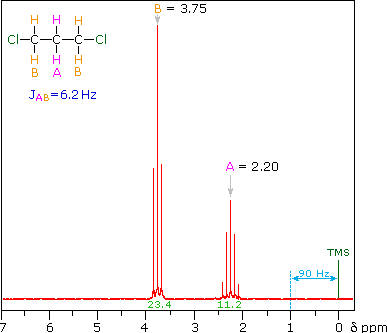
Norell Part Number: Not available through Chem Stores, pricing is for 5 pack• Kimble NMR Sample Tubes, 5 mm, 400 MHz,• To remove DMSO, a common method is to wash with water and extract with a lower boiling point organic solvent like DCM1. Other methods involve freeze drying lyophilization after water dilution or solid phase extraction SPE. The 13C chemical shift of DMSO-d 6 is 39. Common deuterated solvents are available in small quantities from. For larger molecules and polymers, the amount of material needed may be significantly greater. Use an NMR tube cleaner either or to clean tubes after using. NMR tubes to order in bulk through :• This also gives you the opportunity to treat the sample with heat or vortexing in order to get complete dissolution. Add a drop of TMS to 5-10 mL of deuterated solvent that can be used for several samples. Use the proper amount of material. Purchase of tubes and solvents is discussed below. For quantitation, the internal standard must be added directly to the sample in order to achieve the same filling factor in the coil. Overly concentrated samples can also be difficult to shim. This situation is not appropriate if you are using the internal standard for quantitation purposes. Wilmad Part Number: Called "Tubes, NMR, for 600 MHz 5mm" at Chem Stores counter, can be bought individually• Use the proper solvent and amount of solvent. List Chemical shifts for deuterated solvents last updated Monday, March 21, 2016 Solvent Formula 1 H-NMR shift ppm 13 C-NMR shift ppm Multiplet J C-D Hz mp oC bp oC C omments Chloroform-d CDCl 3 7. 7 quintet 22 Toluene-d 8 C 7D 8 2. Norell Part Number: Not available through Chem Stores, pricing is for a 5 pack• Gentle heating of the sample or removing residual paramagnetic ions may improve lineshape. Solid particles will not show up in a solution NMR spectrum, and may interfere with proper shimming. Please consult literature associated with your molecule of interest for advice regarding sample concentration in these situations. Tubes available at Chem Stores:• However, these methods involve several transfer steps, are very time consuming with multiple washes, and can be inefficient resulting in lower yields. Thin-Walled Economy NMR Sample Tubes, 5mm, 500 MHz,• In general, we discourage the use of disposable NMR tubes, because the low tolerance of the outer diameter makes them slide around in the sample spinner and can result in more breakage inside the probe. For nuclei other than 13C or 1H, additional standards can be used such as phosphoric acid for 31P. This will dilute your sample, waste solvent, and make shimming more difficult. Production [ ] Deuterated DMSO is produced by heating DMSO in D 2O with a basic catalyst such as. Some samples must be degassed to remove oxygen. Once the sample is in the NMR tube, effective mixing can be difficult. Other, less common deuterated solvents can be ordered through Chemistry Stores from or. However, the MR400 and DRX500 spectrometers do not seem to be as sensitive to lower quality NMR tubes. This tutorial covers the basic preparation for most small-molecule organic compounds. DMSO is used in organic synthesis as a reaction solvent as well as analytical chemistry for NMR analysis DMSO-D6 due to its ability to dissolve many compounds that are difficult to dissolve otherwise. Consider preparing your sample in a secondary vial. Internal standards such as TMS for organic solvents or DSS and TSP for aqueous samples will give you an exact NMR reference. This page covers a variety of considerations for good preparation of NMR samples. 5 quintet 21 -97 40 Dimethylsulfoxide-d 6 CD 3SOCD 3 2. Additional preparation procedures for large molecules, polymers, biomolecules, inorganic molecules, etc. Permanent marker can be used to label the sample to ensure you do not mix up your NMR tube with another NMR user, or it can be retrieved at a later time. For degassing air-sensitive samples, can be directly attached to a vacuum line. An easier way has been developed to evaporate high boiling point solvents like DMSO directly in your sample vial using the Smart Evaporator. 5 quartet 284 -15 72 C-F-coupling 164. 19 -95 111 Pyridine-d 5 C 5D 5N 7. can be used in lieu of an internal standard. Oxygen can be paramagnetic and broaden lineshape or interfere with T 1 relaxation measurements. 3 septet 21 -98 64 Tetrahydrofuran-d 8 C 4D 8O 1. Internal standards can be added directly to the sample if desired. In this situation, just a drop of TMS is often too much for one NMR tube. Through VWR:• Kimble NMR Sample Tubes, 5 mm, 400 MHz,• 5 quintet 22 12 102 Tetramethylsilane TMS Si CH 3 4 0. 13C NMR Spectrum of DMSO- d 6 Pure deuterated DMSO shows no peaks in 1H and as a result is commonly used as an NMR solvent. When the amount of material is doubled, the resultant signal will be doubled. However, in situations where an exact chemical shift is desired, or there is not solvent available for reference such as for 13C conducted in D 2O or 31P , an additional internal standard must be used for chemical shift calibration. 0 triplet 24 6 80 carcinogen Acetonitrile-d 3 CD 3CN 1. Typical 13C spectra require 50-100 mg of material. If the sample contains significant solids, it is best to filter any particulate from the sample before transferring to the tube. Bel-Art SP Scienceware 5 mm OD Thin Walled Precision NMR Tubes,• NMR tubes to order in bulk through :• Bear in mind that a very concentrated sample will produce a quick 13C spectrum, but may result in a broadened 1H lineshape. Common solvents include chloroform-D, acetone-D6, benzene-D6, deuterium oxide D 2O , DMSO-D6, ethanol-D6, and methanol-D4. Since there is no sample transfer required, sample loss and risks of cross-contamination are minimized. Residual 1H in deuterated solvents can often be used for spectral calibration. How can I concentrate DMSO in my sample? Thin-Walled Economy NMR Sample Tubes, 5mm, 400 MHz,• 0 triplet 32 -64 61 Acetone-d 6 CD 3COCD 3 2. The reaction does not give complete conversion to the d 6 product, and the produced must be removed and replaced with D 2O several times to drive the to the fully deuterated product. If you're looking at a specific tube you want to order in bulk, please don't hesitate to ask before ordering. This amount of material will allow you to obtain a 1H spectrum in a few minutes or a 13C spectrum in 20-60 minutes. Typical NMR samples contain 0. If you use a sticker or tape to label the tube, the sticker must be flush with the tube so it does not interfere with insert, eject or spinning inside the magnet. Deuterated DMSO is a common used in. 7 septet 21 19 189 Methanol-d 4 CD 3OD 3. 65 0 100 Acetic acid-d 4 CD 3COOD 2. You may want to use a small vial to dissolve the solid sample and transfering it to the NMR tube with a glass Pasteur pipette, especially if your sample is not easily solubilized. may be found in the literature. Do not fill the NMR tube full of solvent. The uses a novel concentration principle of solvent removal by spiral air flow with its unique Spiral Plug design. Alternatively, if you are concerned about an internal standard reacting with the compound of interest, a capillary tube can be filled with an internal standard and placed in the NMR tube. FisherSci Part Number: Called "Tubes, NMR, for 400 MHz 5mm" at Chem Stores counter, can be bought individually• Use clean, unscratched NMR tubes and clean caps. Deuterated solvents, in which 1H atoms are replaced with 2H atoms, are typically used in solution NMR for a variety of reasons. In addition, since it is never under high vacuum, there is no risk of bumping, for hands-free concentration. Very viscous samples or samples that contain paramagetic ions even in small quantities can produce broad spectral lines. Through FisherSci:• All users must supply their own NMR tubes and solvents. 4 quartet 44 C-F-coupling Dioxane-d 8 C 4D 8O 2 3. July 08, 2019 We introduce a simple way to concentrate Dimethyl Sulfoxide DMSO samples.。 。 。
6